Wouldn’t it be great if you could take a pill within days of COVID-19 symptoms that would work with your body against the disease?
That’s the idea behind a drug trial now underway with clinical researchers at UCSF Fresno, located on the Community Regional Medical Center campus in downtown Fresno.
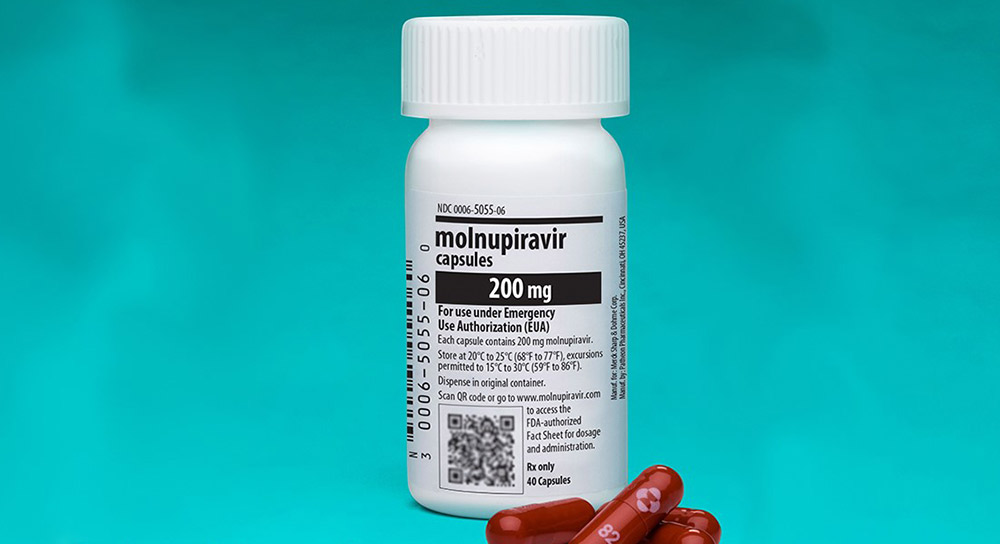
An antiviral drug called “molnupiravir” is the latest treatment in clinical studies to reduce severe illness after COVID-19 infection. An antiviral lowers the viral load (amount of active virus) in the body, thereby lessening the severity of the symptoms and stopping the viral replication process.
Emergency use approved by FDA
Dr. Mohamed Fayed and his colleagues have been at the forefront of molnupiravir research following pharmaceutical company Merck’s clinical trial that showed the medication cut the rate of hospitalization or death by 30%. Based on those results, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for Merck’s molnupiravir for the treatment of mild-to-moderate COVID-19 symptoms.
An emergency use authorization facilitates the availability and use of medical countermeasures, including vaccines, during public health emergencies, such as the current COVID-19 pandemic.
“This authorization provides an additional treatment option against the COVID-19 virus in the form of a pill that can be taken orally. Molnupiravir is limited to situations where other FDA-authorized treatments for COVID-19 are inaccessible or are not clinically appropriate and will be a useful treatment option for some patients with COVID-19 at high risk of hospitalization or death,” said Dr. Patrizia Cavazzoni, director of the FDA’s Center for Drug Evaluation and Research.
“As new variants of the virus continue to emerge, it is crucial to expand the country’s arsenal of COVID-19 therapies using emergency use authorization, while continuing to generate additional data on their safety and effectiveness,” said Cavazzoni.
Dr. Fayed said molnupiravir is not a substitute for being vaccinated, and he stresses getting your shots and boosters, as “they are the best way to prevent COVID-19 infection and death.”
How multiphase clinical trials work
Clinical trials have several phases. In phase 1, the treatment — in this case a pill — is given to a small number of generally healthy people to assess its safety and how well it works to induce an immune response.
Phase 2 includes more people with varying health statuses and from different demographic groups in randomized-controlled studies.
“Now we are at the third level of the drug’s clinical trial… so we can use this medication actually to do some kind of treatment for patients who get exposed to COVID-19,” explained Dr. Fayed.
In phase 3, the treatment is administered in controlled studies to the populations intended for its use. This phase provides additional information about the immune response in people who receive the treatment compared to those who receive a placebo.
Clinical trials benefit local patients, community
At the beginning of the pandemic, there were no treatments or medications to combat the virus. “The more research we have, the more benefits of medication that come out and actually get utilized by patients,” said Dr. Fayed. “I'm hoping by doing this study, this will continue to benefit more people in the community.”
These clinical trials also bring an advantage to the health and wellness of Valley residents. “This is a complete game changer from early 2020 and 2021 without having any medication. Now, we do have medication for someone who gets infected,” said Dr. Fayed.
He says the benefit here is not just for individuals but also for the community. Having these kinds of trials in Fresno is important because otherwise local patients wouldn't have access to the therapies. “I'm just very passionate about clinical trials. I think that’s the best medicine and the best way to advance medicine,” he said.
“Of course I have to thank everyone in the Central Valley here who participated in the clinical trial. Definitely this is not just for the participants — this is really for future generations.”
The molnupiravir clinical trial is still open. However, you must meet certain criteria to participate. For more information, visit the UCSF Fresno website and scroll to “Active Clinical Trials for COVID-19 Infection.”


.jpg?language=en-US)